Grade 7 First Final Term Revision 2022 Chemistry
Grade 7
Chemistry
Question 1
The particles move from an area of
low concentration to an area of high concentration is called diffusion. F
Molecules are groups of 2 or more
atoms bonded together. T
Elements contain only one type of
atom. T
Compounds contain 2 or more types
of atom. T
Chemists use a kind of short-hand
to describe molecules. It's called the symbol. F
Question 2
The particles move from an area of ?? concentration to an
area of low concentration is called diffusion. High
Good scientists make sure their data provides evidence that
is reliable and ?? . valid
You can use existing theories to explain??. Ideas
??tells us how near the true reading your measurement is. Accuracy
I'm Elvis the Electron and I'm pretty quick, I fly round the
nucleus at a fair old lick! The protons and I, we tend to attract, I'm ?? you
see and that's a fact! Negative
Question 3
1 There are 3 types of particle inside an atom.
a)Protons b)neutrons c)electrons
d)ALL
2 protons and neutrons have the same
b)Charge c)color d)smell
a)Mass
3 The first shell (nearest to the nucleus) can hold just
------- electrons.
a)4 c)5 d)6
b)2
4 the number of protons + the number of neutrons =
a)Atomic size b)atomic
number d)atomic charge
c)Mass number
5 lsotopes are atoms with the same number of protons, but
different numbers of------.
a)Proton b)electron
d)none of them
c)neutrons
A 10 in A 0 in B
b 0 in A 10 in B c 8 in A 2 in B
d 5 in A 5 in B
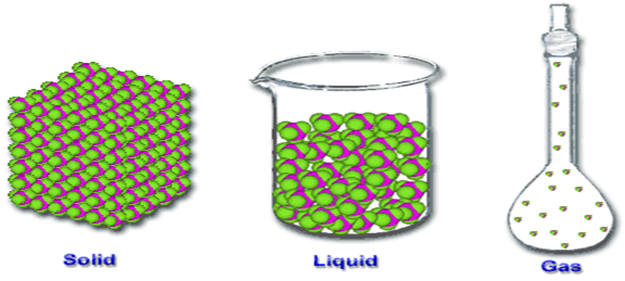
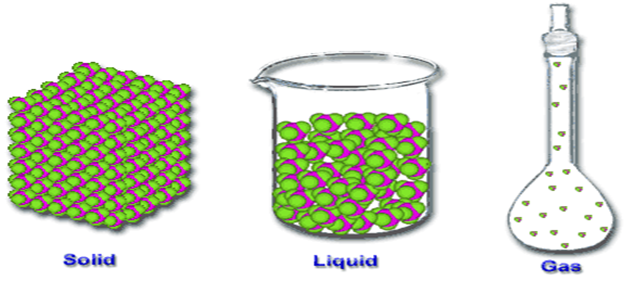
------------------has definite shape and volume
A Gas b Liquid d All of above
C Solid
----------------------- is an example of atom.
B H2 c
H2O D none of them
A He
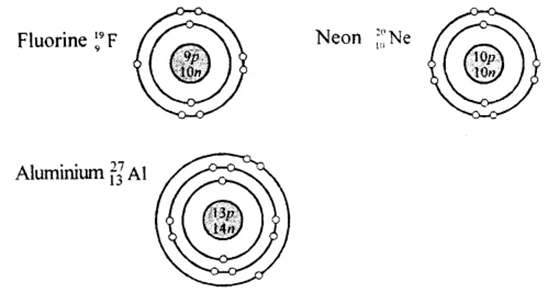
Cl is the symbol of
A Fluorine b
Neon c
Aluminium
D none of the above
In ammonia number of Nitrogen is
B 2 C 3 d 4
A 1
----------------------- is an example of element.
A both c H2O D
none of them
B H2
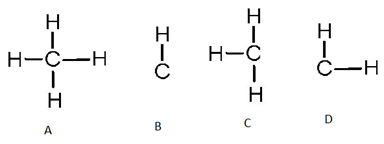
Formula of CH4 is
B B
C C D D
A A
Is an example of
B unbalance
equation c Both of them d
none of them
A balance equation
State symbol of gas is
A (s) B (l)
D All of the above
C (g)
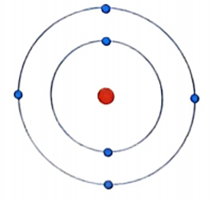
In this diagram -------------- color show nucleus
A blue c green d yellow
b Red
Electrons carries ----------------- charge
A positive c no d both a & b
B negative
In 1st shell number of electrons---------------
B 4 C 6 D 8
A 2
Is an example of ---------------------
A Helium B sodium
c Hydrogen
D Carbon
Atomic number of Helium is-------------
A 1 C
3 D 4
B 2
Atomic mass of this element is
A 12 B
18 C 20
D 24












Comments
Post a Comment